Presentation
Raybow provides expert contract process development & manufacturing. 2,000 employees can accommodate a variety of chemistries, offering high quality services designed to accelerate the drug development process from pre-clinical to commercial launch. Raybow manufactures advanced intermediates, regulatory starting materials and APIs in US FDA inspected facilities in Taizhou & Suzhou, China and Brevard, USA. Raybow also has state-of-the-art PR&D centers in Brevard, USA, & Hangzhou & Taizhou in China.
Thank you for your interest in Raybow PharmaScience. Our North American headquarters are located in Brevard, North Carolina. Our Brevard site offers Chemistry Development Services including:
- Custom Synthesis
- Synthesis Route Development
- Process Development
- Analytical Method Development and Validation
- Extensive on-site analytical instrumentation
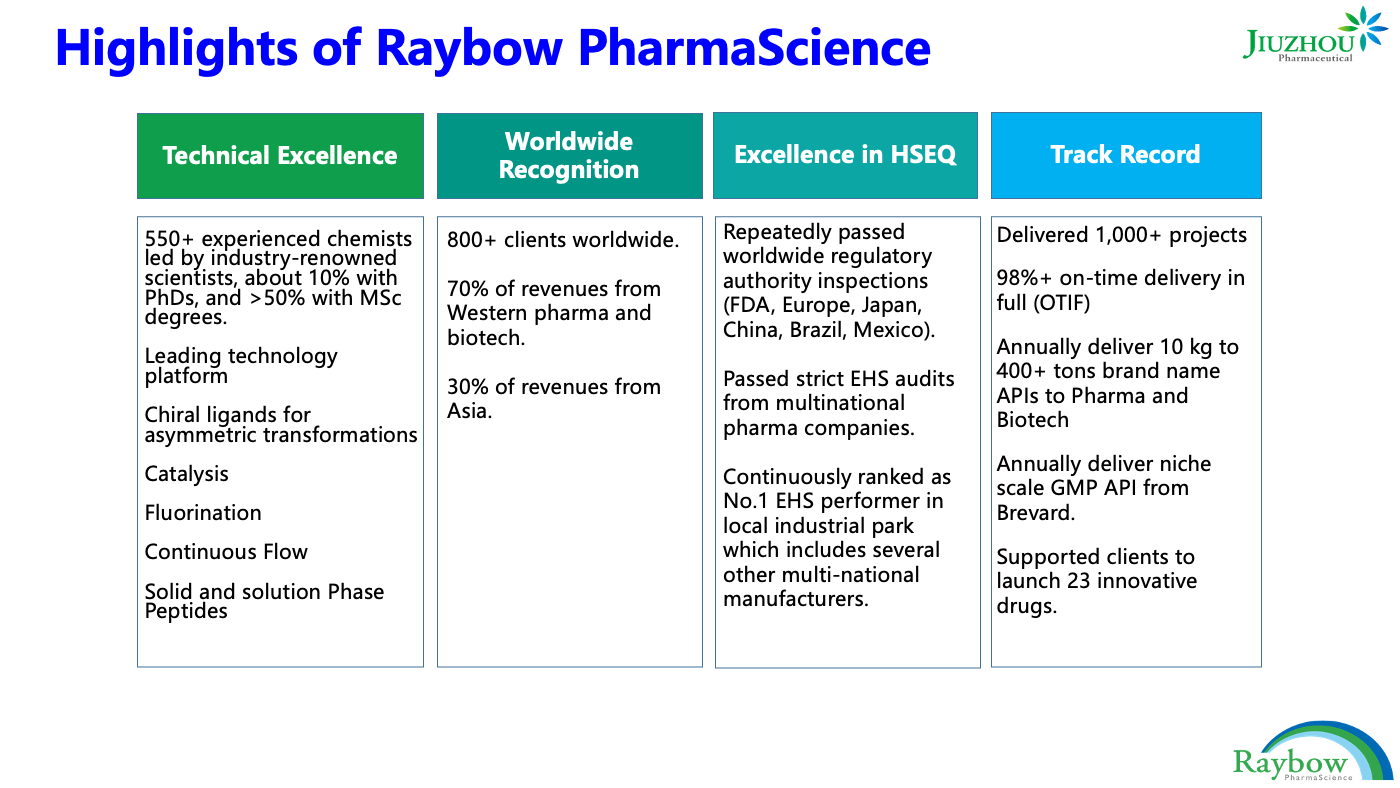
Global operations include 450+ R&D chemists dedicated to new discoveries and innovation and 2000+ dedicated employees and growing who have delivered 1000+ projects with a 98% on-time delivery record. Please contact us today to learn how we can help get your project to market.
Raybow News & Papers
Raybow announces $15.8 million expansion of U.S. site, tripling capacity and U.S. workforce
First of all, Green Chemistry is “the development of chemical products and processes to reduce or eliminate the use and generation of hazardous substances” and “is intended to apply to the development and full lifecycle of a chemical product” i.e. development, manufacturing, application and disposal.