Comprehensive CDMO/CMO Partnerships
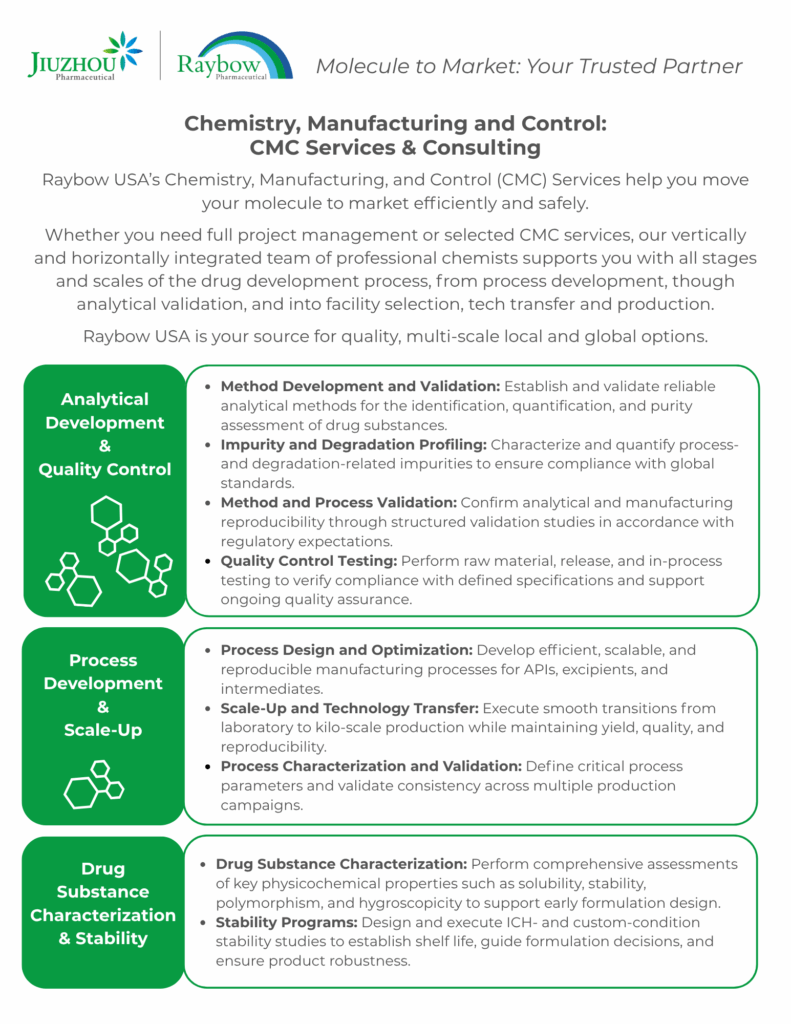
Raybow USA offers Research & Development and Analytical Services, with cGMP and nonGMP production capabilities in pharmaceutical and specialty markets. Check out the At a Glance page for more information about the company and its capabilities, including Specialty Chemicals.
Preclinical Study (CMC)
Process design and screening
Verification of process parameters, safety and quality attributes
Preparing of IND filing batch and IND filing support
Clinical Stages
Technical transfer
Process optimization for COGS, Starting materials and Process Robustness
Production of clinical APIs
Kilogram production
Long-term and Accelerated Stability Studies
Continuous optimization
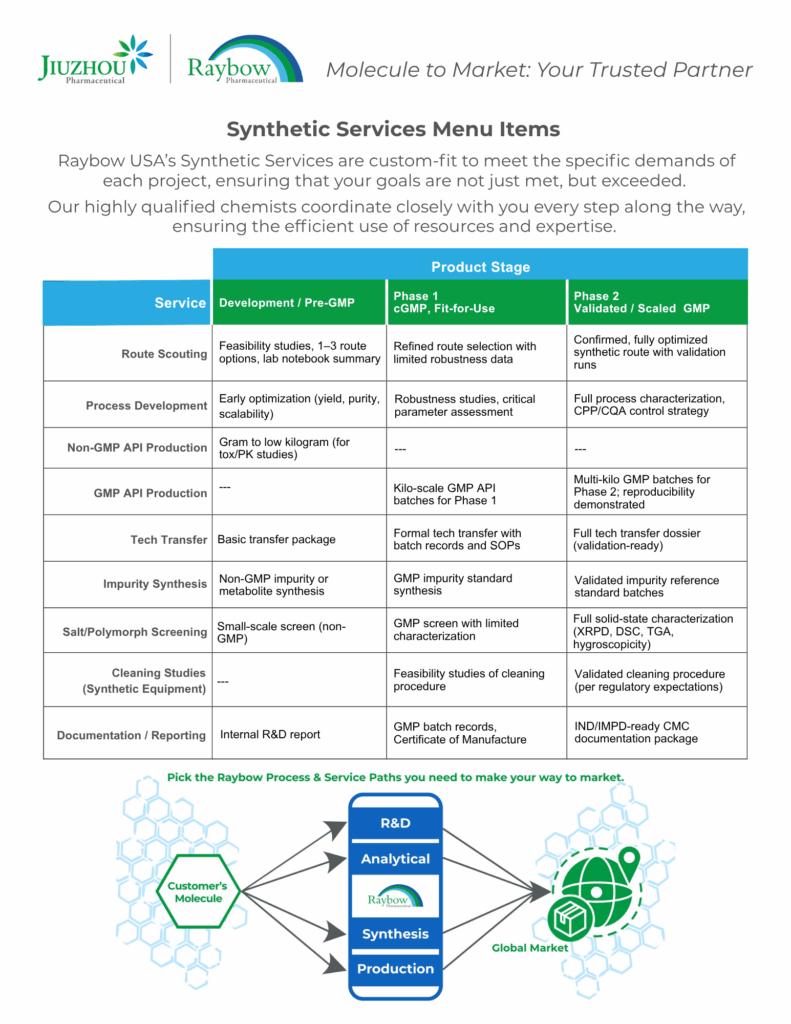
View Raybow USA's Services Menus for Sample Packages and Services
As part of the Jiuzhou Pharma global CDMO, Raybow USA offers customers access to green chemical manufacturing through all stages of production, with facilities and chemists in the United States, Europe, Japan and China.